Sampling and submission
Sample Submission
If you would like to send samples to us, then please contact the relevant theme leads (below) who will request further details. Once the work has been discussed and agreed they will advise on the sampling protocols, permits and packaging requirements, prior to shipment.
Crustacean materials: kelly.bateman@cefas.co.uk
Fish materials: richard.paley@cefas.co.uk
Molluscan materials: frederico.batista@cefas.co.uk
Please note: Unsolicited samples received by the Collaborating Centre will not be opened, analysed or otherwise assessed. In these instances, samples and their packaging materials will be discarded as appropriate. Please ensure you contact us before shipping any materials.
Sampling
Sampling protocols are available upon request, please contact the relevant theme leads:
Crustacean materials: kelly.bateman@cefas.co.uk
Fish materials: richard.paley@cefas.co.uk
Molluscan materials: frederico.batista@cefas.co.uk
Responsibility to Report to Competent Authority
The OIE Collaborating Centre for Emerging Aquatic Animal Disease requires each sample submission to be reported to the relevant Competent Authority within the Country of origin prior to shipments of materials. Should we find a notifiable disease within submitted samples we will request that this is reported to the OIE via the Competent Authority of the country of origin.
Please note: Should the Competent Authority neglect to inform the OIE directly within a 2-week period (from receipt of confirmed results) we are obliged to report the findings on behalf of the country of origin. By submitting samples to the OIE Collaborating Centre for Emerging Aquatic Animal Disease we will assume agreement and compliance in this matter.
Import Permits
Samples may require an import permit from the Animal and Plant Health Authority (APHA); these must be applied for prior to shipment of any materials to the UK. Staff at the OIE Collaborating Centre for Emerging Aquatic Animal Disease will advise and assist with all applications.
Please note: No samples should be submitted to the OIE Collaborating Centre for Emerging Aquatic Animal Disease without prior agreement to enable import permits etc to be supplied.
Nagoya Protocol
The UK is a ratified party to the Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization. In order to comply with this protocol the OIE Collaborating Centre for Emerging Aquatic Animal Disease requires that all sample submissions are reported to the relevant ABS Focal Point prior to shipment of materials. Should there be a requirement to provide a Material Transfer Agreement (MTA) or any other related documents please contact the relevant theme leads.
Please note: No samples should be submitted to the OIE Collaborating Centre for Emerging Aquatic Animal Disease without notifying the relevant ABS Focal Point from the country of sample origin.
Packaging and Shipment
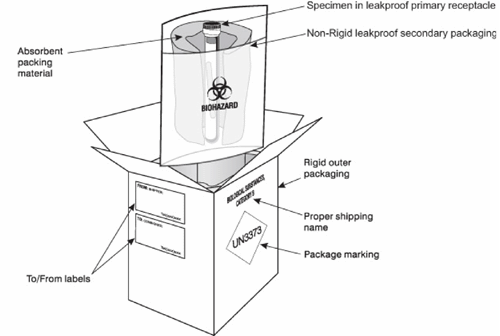
All samples must be packaged to comply with the transport of infectious substances, UN3373, Biological Substance, Category B. Further advice on packaging and shipment is available upon request, please contact the relevant theme leads.
Example of Packing and Marking for UN3373, Category B Infectious Substances (recreated from royalmail.com)